Introduction
Many women can not wear certain items of jewellery because the metal causes an allergic reaction on contact with the skin. The offending metal most often is nickel, but really most metals can cause allergic reactions. These same metals are often used in dentistry without any consideration as to whether a patient has an allergy. The problem is contact allergies are fairly rare in the mouth because the metal ions dissolve in the saliva and then pass through the gut into the body. Instead of getting a skin rash, the problem shows up somewhere else in the body, potentially as a full blown autoimmune disease. In the paper Investigation of contact allergy to dental metals in 206 patients they found that 13% had a contact allergy to metals commonly used in dentistry. But only 7% of the patients had lichen planus, or a contact allergy in the mouth. Officially there is no known cure for oral lichen planus, but upon removing the offending metal the problem goes away.
Metal Allergy Test
The Melisa Foundation have a blood test called the MELISA (Memory Lymphocyte Immunostimulation Assay). It can test specifically whether a person is allergic to a certain metal, and how great that immune response is. It has been used to successfully identify allergies and removing the offending metal.
The MELISA® Medica Foundation has conducted extensive search on CFS patients. A study involving 930 fatigued patients saw more than half (62 percent) test positive for metal allergy. The majority of those who went on to remove the offending metal reported substantial health improvements.
Media
This is a lecture from the woman that invected the MELISA test.
Lead And Arsenic
Many of the dental crowns and bridges outsourced to China have recently been found to contain high amounts of lead and arsenic. This has been picked up upon by the news media, but nothing has been done about it. There is currently no regulation or testing required for imported dental work.
When this new report was released the FDA responded by saying:
"The ADA is taking this report very seriously."[1]
"There is no appropriate use for lead in manufacturing dental prosthetics."[1]
A year later they released a report saying:
"The results ranged from below detectable to 113 parts per million (ppm) in the 44 porcelain powders, and an average of 46 ppm in the 102 porcelain dental crowns. "[2]
So they agreed with the news report that these crowns did indeed contain lead. Then they went on to say:
"ADA Laboratory Tests Find
Lead Not Released From Dental Crowns
Crowns subjected to testing conditions far more extreme than found in
mouth"[2]
The extreme conditions that they claim consisted of heating the teeth to 80 degrees C in a solution of a weak acid for 16 hours. These extreme conditions apparently didn't include chewing, or exerting any kind of abrasive force upon the crowns. The woman in Ohio from the news report., her dentist drilled on her crowns potentially releasing large amounts of lead into her body. If drilling releases heavy metals from these crowns, surely chewing would do the same ?
[1] http://web.archive.org/web/20080312083145/http://www.ada.org/public/media/releases/0802_release05.asp
[2] http://www.ada.org/3268.aspx
Uranium
Although this section is mostly historic, it is interesting to know that uranium was deliberately added to porcelain, up until the 1980s. The idea was that the fluorescence of the uranium would help mimic the look of real teeth under a variety of natural and artificial light conditions. As well as being radioactive, uranium is also highly toxic, and no doubt contributed to an unnecessary body burden to the patient. Although there were no direct studies that proved it was dangerous in dental materials, the companies that made these products were smart enough to phase out the metal in favour of safer alternatives.
The following image from reddit user 33mmeyes shows natural and porcelain teeth of today against a uv light. The natural teeth glow, whilst the porcelain ones do not.
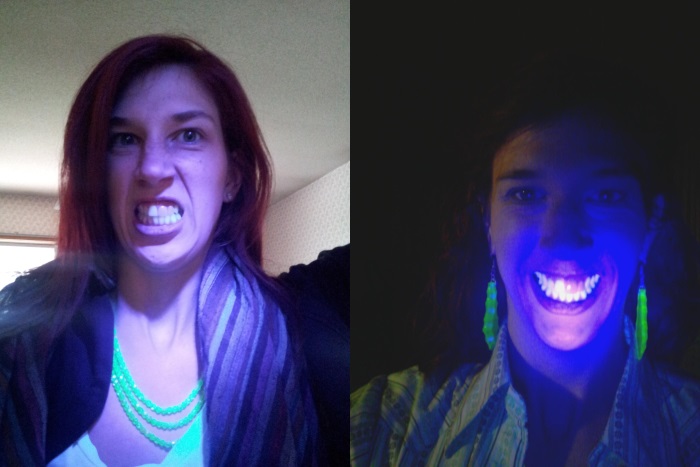
[ Image source: http://imgur.com/a/lcmSI]
Further reading:
https://www.orau.org/ptp/collection/consumer%20products/dentures.htm
Galvanic Corrosion
Galvanic corrosion can happen when metals with different eletrical potentials are put together in an electrolyte solution. The metal ions will migrate from one metal, to the other. This same process also happens in batteries to produce electricity. It can also happen in the mouth. Dentists will routinely use various metals in a patients mouth without any regard for the different eletrical potientials of the metals. The release of mercury in dental amalgam can be greatly accelerated by this process, especially when in contact with gold[1].
The following video shows it is possible to make a battery using metals commonly used in dental restorations.
[1] http://orthomolecular.org/library/jom/1989/pdf/1989-v04n03-p141.pdf